Scientific Highlights from the PTCOG Subcommittees
- Breast Subcommittee
Update on the Radiotherapy Comparative Effectiveness Study (RadComp)
The RadComp study is a pragmatic randomized clinical trial comparing two types of radiation in locally advanced breast cancer and funded by the Patient-Centred Outcomes Research Institute. The primary objective of the study is to assess the effectiveness of proton vs. photon therapy in reducing major cardiovascular risks. Secondary outcomes include assessing the non-inferiority of cancer control and health-related quality of life measures between the two treatment arms. Participants are stratified by age (<65 vs >65), cardiovascular risk (0-2 vs >2 risk factors), surgery (mastectomy vs lumpectomy) and treatment laterality (left vs right).
The study is nearing its target enrolment and anticipates it will finish enrolment in 2024. As of 31 December, 2023 over 100 investigators enrolled 1,224 participants from 30 proton networks across the country.
In addition to finishing enrolment in 2024, the study will begin a transition to a decentralized model for long-term follow-up. Shifting to a decentralized model will offer more flexibility for the study’s participants and reduce burden on local site research teams.
For more information about the RadComp study, please contact Hien Lu at

******************************************
Proton Minibeam Radiation Therapy (pMBRT)
Proton Minibeam Radiation Therapy (pMBRT) is an innovative RT approach that uses a distinct radiation dose delivery method. Irradiation is performed by using an array of parallel thin (0.5- 1 mm wide) beams spaced by 1 to 4 mm. The use of narrow beams allows to exploit the dose volume-effect: the smaller the beam size is, the higher the tolerance of normal tissues. A combination of proton beams and this delivery method offers the possibility to (a) maintain dose spatial fractionation at the entrance of the beam and in the beam path; (b) produce a more uniform dose than x-rays in a target at depth (where the multiple scattering of protons makes a wider minibeam); (c) achieve a higher dose at any depth than in the path; and (d) preserve tissues after the target by the inherent property of a determined range of proton beam. In preclinical studies we have already proven the ability of pMBRT to (i) drastically reduce neurotoxicity and (ii) provide an equivalent or superior tumour control than standard PT. The resulting outstanding increase in the therapeutic index opens the possibility for more aggressive irradiation schemes than the standard ones. pMBRT notably allows to irradiate large areas of the brain or even whole brain irradiation without major toxicity, which can be advantageous for glioma treatments to face their infiltrative nature.
The first technical implementation of pMBRT at a clinical centre was achieved at the Institut Curie-Orsay Proton Therapy Centre (ICPO) in 2014. Since then, a series of radiobiological studies were carried out. In an in vivo pioneering experiment, pMBRT demonstrated to lead to a remarkable gain in normal (brain and skin) tissues tolerances. Long-term follow-up of rats receiving whole-brain irradiation with either standard PT (25 Gy in one fraction) or pMBRT (peak doses of 58 Gy/one fraction, average dose of 25 Gy) revealed no significant damage after pMBRT treatments while extensive brain damage, including necrosis, demyelination, astrogliosis and microglial activation, was observed in conventional PT irradiated group. It has also been demonstrated that pMBRT has no significant impact on cognitive, motor or emotional processes. Skin toxicity reduction has also been confirmed with low energy proton beams at research facilities. The biological data gathered so far in pMBRT encourages the preparation of phase I clinical trials.
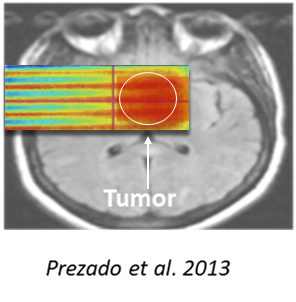
Currently new dosimetric proof of concept studies are running in different indications as pMBRT in breast tumours in comparison with SBRT, as well as cardiac ablation of ventricular tachycardia. This beautiful story of research and clinical implications is to follow.
Yolanda Prezado and Youlia Kirova, Institut Curie, Paris, France
*******************************************************
- BNCT Subcommittee
- Dr. Hiroshi Igaki was recently elected President of the Japanese Society for Neutron Capture Therapy. He is head of the Department of Radiotherapy at the National Cancer Centre Hospital in Tokyo and is responsible for the clinical trial of BNCT for malignant skin tumours. http://www.jsnct.jp/e/index.html
- The University of Tsukuba is preparing a phase I trial with BNCT for the treatment of glioblastoma. The protocol is currently being reviewed by the PMDA (Japanese Medicines and Medical Devices Agency). https://www.pmrc.tsukuba.ac.jp/research/en/index.html
****************************************************
- Early Career Researcher Subcommittee
The PTCOG Early Career Researcher (ECR) subcommittee was established during the 61st PTCOG conference. We are a dedicated group representing the interests of emerging researchers and practitioners in particle therapy. Our mission is to foster collaboration, provide support, and address the needs of those navigating their early careers. This includes:
- PhD, Master’s, and Undergraduate Students
- Postgraduate and Postdoctoral Researchers
- Medical Physicists in Training
- Physicians in Training and Residents
- Other Early-Career Scientists

Join the PTCOG-ECR community:
Are you an ECR interested in PTCOG-ECR activities? Join us!
As a member, you’ll have the opportunity to propose ideas, participate in activities, and contribute to shaping the future of particle therapy, while benefiting from a supportive network of peers.
PTCOG-ECR Activities:
- Slack Networking Platform: We are excited to announce the launch of our new Slack platform! This will serve as an interactive hub for networking and knowledge sharing within the ECR community.
- PTCOG-ECR plenary session at PTCOG: Join us in Singapore for the inaugural ECR plenary session - “Open-Source Thinking: The Future of Data Sharing for Medical Research”
- Newsletter: Sign up to receive the quarterly ECR newsletter, with subcommittee news, scientific insights, job opportunities, and event information.
- ECR Social and Mentoring Events at PTCOG 62: Stay tuned for updates!
Connect with us:
Don’t hesitate to
If you have ideas or suggestions for the subcommittee, please let us know using the PTCOG-ECR new ideas form.
Looking forward to hearing from you,
The PTCOG-ECR Co-Chairs and Operative Group
Bethany Rothwell, Ph.D.
Massachusetts General Hospital
Gabriele Parisi, Ph.D.
University of Pavia, IRSN
Giorgio Cartechini, Ph.D.
University of Miami, USA
2023/2024 Operative Group:
Elena Fogazzi
University of Trento
Gaia Franciosini
Sapienza University of Rome
Hannah Cook
National Physics Laboratory
Isabella Colizzi
Paul Scherrer Institute
Jeremy Khong
The Christie NHS Foundation Trust
Nadine Vatterodt
Danish Center for Particle Therapy
Anastasiia Quarz
GSI & TU Darmstadt
Valeria Pascali
University of Pavia
Daniel Ebner
Mayo Clinic
Marco Battestini
University of Trento
****************************************************
- Gastrointestinal GI Subcommittee
Paper Announcement:
Lower Gastrointestinal Tract Malignancies Consensus Statement: Proton Therapy Indications, Treatment Delivery and Case Discussion by Choi JI, Wojcieszynski A, Amos R, Giap H, Apisarnthanarax S, Ashman JB, Anand A, Perles L, Williamson T, Ramkumar S, Molitoris J, Simone CB 2nd, Chuong MD was accepted for publication in Int J Part Ther. 2023
**************************************************
- Genitourinary GU Subcommittee
Promising Long-Term Outcomes Following Proton Therapy for Localized Prostate Cancer
The delivery of proton therapy for localized prostate cancer remains one of the most controversial subjects in radiation oncology. The lack of long-term data is often cited as one of the reasons for persistent scepticism among radiation oncologists. A study recently published in Radiotherapy and Oncology provides much anticipated long-term data from MD Anderson, documenting outcomes following proton therapy for localized prostate cancer.
The study’s cohort was derived from two prospective registries that included 2,772 men with localised prostate cancer treated between 2006 to 2020. Most of the patients were treated with conventionally fractionated proton therapy (98.9%) with total doses that ranged between 72 and 79.2 Gy(RBE) delivered at 1.8 to 2.4 Gy(RBE) per fraction. Androgen deprivation therapy was up to the discretion of the physician and was given to a total of 1,562 (56%) men for a median of 6 months for patients with unfavourable intermediate risk disease and 24 months for patients with either high risk or very high-risk prostate cancer. Patient disease control outcomes were stratified by the National Comprehensive Cancer Network risk (NCCN) categories and physician reported toxicity was reported using a modified scale based in part on the Radiotherapy Oncology Group (RTOG) scale.
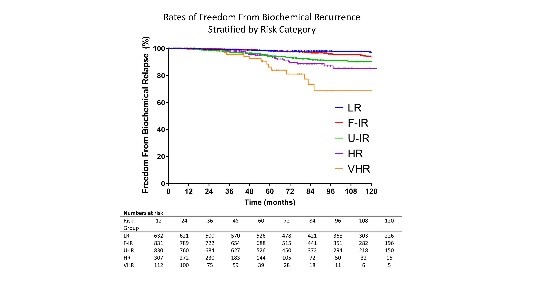
With a median follow up of 7 years, the 10-year freedom from biochemical relapse was 96.8%, 93.6%, 90.2%, 85.2% and 68.5% for patients with low risk, favourable intermediate risk, unfavourable intermediate risk, high risk and very high-risk disease, respectively. On multivariate analysis, significant predictors for biochemical failure included patient age, T stage, PSA level, Gleason score and NCCN risk group. Late grade 3+ genitourinary and gastrointestinal toxicities occurred in 0.87% and 1.01% of patients respectively.
These results among others from University of Pennsylvania, University of Florida, and the Proton Therapy Centre at the Sapporo Teishinkai Hospital in Japan, provide good benchmark outcomes for future comparative studies between proton therapy and other modern treatments and techniques. They also provide support for the continued delivery of proton therapy for localised prostate cancer as the community awaits the results from the COMPPARE (NCT03561220) and PARTIQoL (NCT01617161) trials.
Curtis Bryant, UFHealth, Florida and Seungtaek Choi, MD Anderson, Texas
**********************************************
- Thoracic Subcommittee
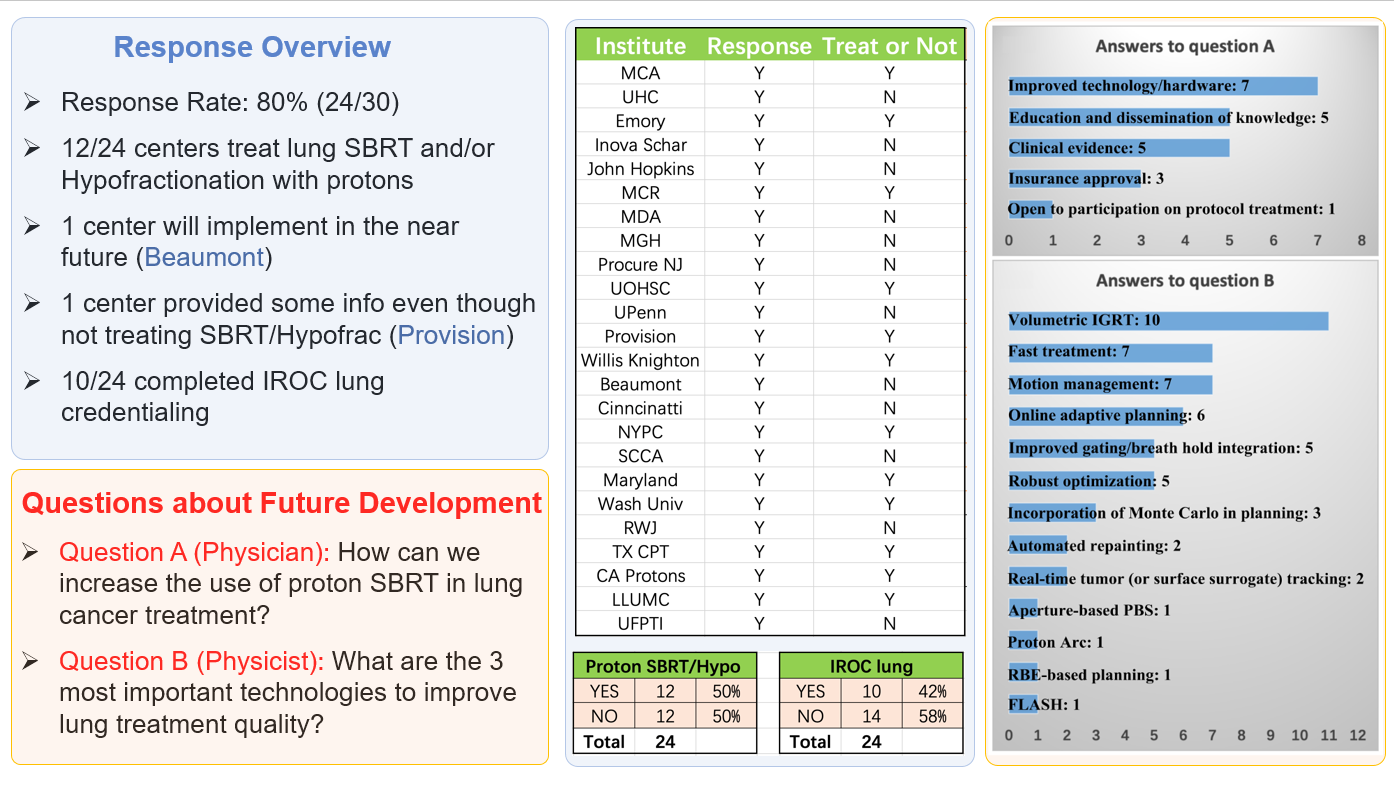
The publication of “NRG Oncology and PTCOG Patterns of Practice Survey and Consensus Recommendations on Pencil-Beam Scanning Proton Stereotactic Body Radiation Therapy and Hypo fractionated Radiation Therapy for Thoracic Malignancies” is accepted for publication in the International Journal of Radiation Oncology, Biology, Physics.
ABSTRACT:
Stereotactic body radiation therapy (SBRT) and hypofractionation using pencil-beam scanning (PBS) proton therapy (PBSPT) is an attractive option for thoracic malignancies. Combining the advantages of target coverage conformity and critical organ sparing from both PBSPT and SBRT, this new delivery technique has great potential to improve the therapeutic ratio, particularly for tumours near critical organs. Safe and effective implementation of PBSPT SBRT/hypofractionation to treat thoracic malignancies is more challenging than the conventionally fractionated PBSPT due to concerns of amplified uncertainties at the larger dose per fraction. NRG Oncology and the Particle Therapy Cooperative Group (PTCOG) Thoracic Subcommittee surveyed US proton centres to identify practice patterns of thoracic PBSPT SBRT/hypofractionation. From these patterns, we present recommendations for future technical development of proton SBRT/hypofractionation for thoracic treatment. Amongst other points, the recommendations highlight the need for volumetric image guidance and multiple CT-based robust optimization and robustness tools to minimize further the impact of uncertainties associated with respiratory motion. Advances in direct motion analysis techniques are urgently needed to supplement current motion management techniques.
Wei Liu, Mayo Clinic College of Medicine and Science Phoenix, Arizona
******************************************************
- Treatment Efficiency Subcommittee
Proposal to (redefine the) self-perception of the PTCOG Treatment Efficiency Subcommittee
Purpose
Fighting cancer effectively and efficiently
Vision
As an integral part of PTCOG, our ultimate goal is to establish, develop, and improve particle therapy modalities and their workflow applications in such a way that the treatment of our patients can be delivered and assured in the utmost efficient way in terms of quality and safety.
Mission
Concrete definitions of efficiency are highly domain specific. In general, efficiency can be interpreted as achieving maximum productivity with minimum effort or cost. In our context, we understand:
- maximum productivity to mean safe, effective, and innovative treatment - not simply more treatments - and
- minimum effort or cost to be the selection of the best modality for resource-optimized application of the intended irradiation.
Based on these considerations, we focus our activities on workflow efficiencies, which should allow for
- accurate and realistic planning of the irradiation dose in a reasonable time frame,
- together with the safe and effective application of the calculated dose distribution,
- aiming at imaging, measurement, and control of predefined application conditions,
- accompanied by comprehensive QA measures before, during, and after irradiation,
- to mitigate and/or minimize any risk to the patient.
Objective
The main factors impacting workflow efficiencies ultimately result from the particular characteristic particle properties we use for tumour irradiation and the resulting, clinical expectations in the context of the latest technical developments. Therefore, we see our role in sharing and transferring knowledge about the following influencing factors on workflow efficiencies according to our mission statement.
- Complexity of workflow-driven QA (general & patient-specific)
- Delineation tools
- Patient immobilization and couches
- Treatment planning processes and systems
- Dose calculation algorithms
- Motion management strategies
- Image guidance
- Beam delivery
- Level of timely treatment adaptation capability (adaptive proton or particle therapy)
- Overall integration / Workflow management
- Data connectivity
- (Lack of) standardization (due to limited number of treatment sites)
Frank Emert, Paul Scherrer Institut, Switzerland, (

Good Clinical Proton Practices (GCPP)
Good Clinical Proton Practices (GCPP) is an initiative that was launched during PTCOG 2022 to facilitate open, dynamic, and organized communication within PTCOG and between proton centres of different stages of development on the exchange of clinical proton practices of different types used in the daily treatment of patients. Knowing that proton treatment in the clinic on real patients is conceptually different from purely scientific proton research, the goal is to initiate an interactive, easily accessible platform that any proton centre could have access to in order to receive, discuss and develop established proton treatment standards. Ideally, this could lead to (scientific) GCPP standards suitable for dissemination or publication and could provide a basis for exchange between different PTCOG SCs.
Robabeh Rahimi, Inova Health, Fairfax, VA, (

*****************************************